Thermal energy is the total kinetic energy of the particles (atoms and molecules) in a substance due to their random motion.
Heat is the transfer of thermal energy from one object or substance to another due to a temperature difference. Its SI unit is joule(J) and CGS unit is calorie (Cal). Heat is transmitted by the methods of conduction, convection and radiation. Heat flows from a body at a higher temperature to a body at lower temperature until both attain the same temperature.
The total amount of heat present in a body is directly proportional to the mass of the body and average kinetic energy of the molecules of that body.
Relationship between calorie and joule
1 calorie = 4.2 joule
One calorie is defined as the amount of energy required to raise the temperature of 1 gram of water by 1 degree Celsius (°C) at a pressure of 1 atmosphere.
One joule of heat is defined as the amount of energy required to raise the temperature of one kilogram of a pure substance (having a specific heat capacity of 1 J/kg°C) by one Kelvin (K) or one degree Celsius (°C).
Temperature is a measure of the average kinetic energy of the particles in a substance. Its SI unit is kelvin (K). But temperature is commonly measured in degree Celsius (°C) and degree Fahrenheit (°F).
Effects of heat on volume of an object
When heat is applied to an object, it typically causes the object to expand. This expansion happens because heat increases the kinetic energy of the particles (atoms or molecules) within the object, causing them to move more rapidly and occupy more space. On contrary, when an object loses heat, the opposite of thermal expansion occurs: thermal contraction. This is a decrease in volume as the particles within the object lose kinetic energy, move less, and come closer together. Therefore, the volume of solid , liquid and gas increases on heating and decreases on cooling.
Anomalous Expansion of Water
Anomalous expansion of water refers to the unique behavior of water in which it expands rather than contracts as it freezes. Specifically, as water cools from 4°C to 0°C, it increases in volume due to the formation of a crystalline structure (ice) that is less dense than the liquid water. This results in ice being less dense than liquid water, causing ice to float.
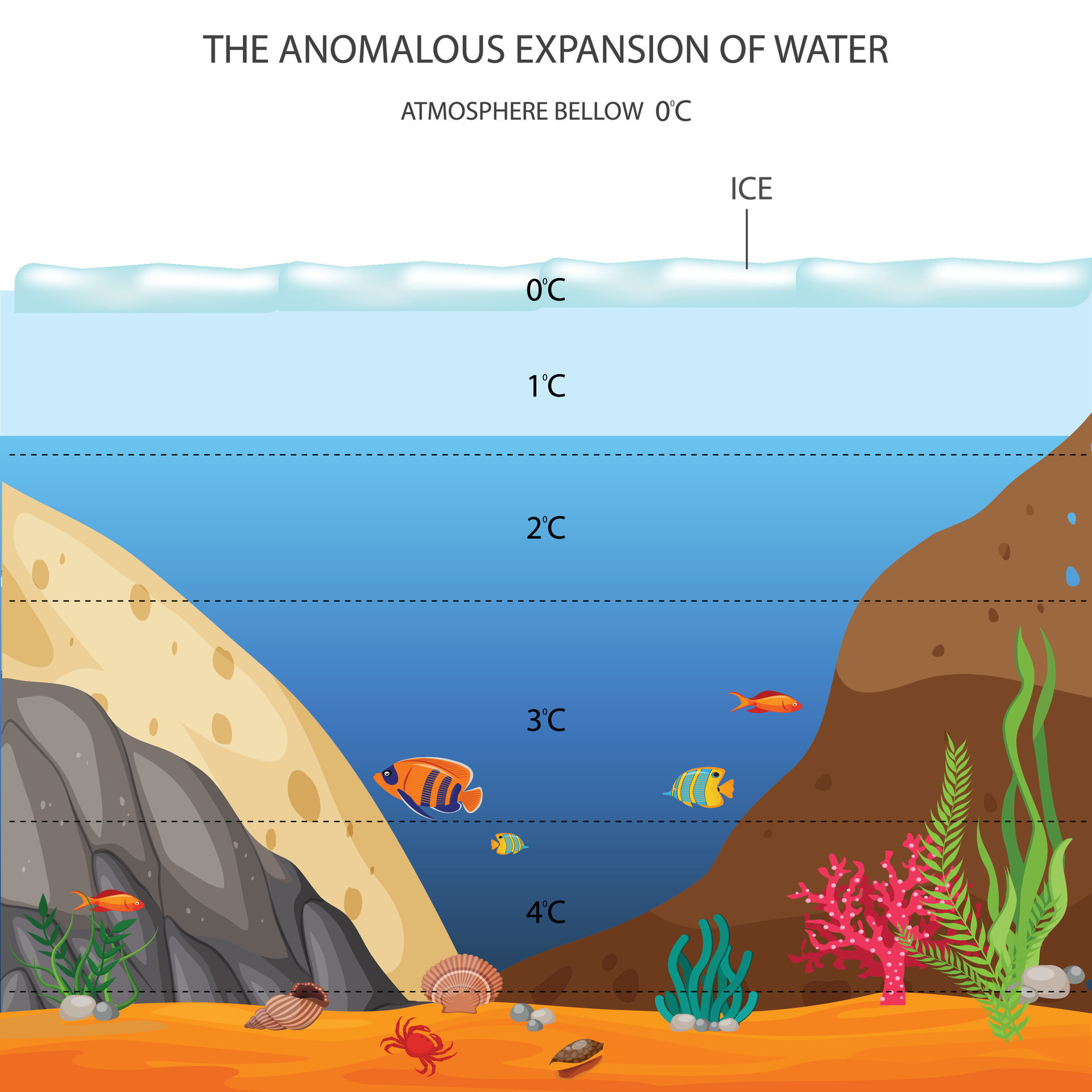
Advantages
- Ice forms an insulating layer on the surface of bodies of water, protecting aquatic organisms from extreme cold temperatures and preventing the entire body of water from freezing solid.
- The formation of ice on oceans and lakes helps to regulate Earth’s climate by influencing heat exchange between the atmosphere and the ocean.
Disadvantages
- Water pipes burst in the winter due to the anomalous expansion of water when it freezes. When the temperature drops and water inside a pipe freezes, it expands. This increase in volume puts immense pressure on the walls of the pipe. If the pressure exceeds the pipe’s capacity to contain it, the pipe will crack or burst. The resulting break can lead to water damage and flooding once the ice thaws and the water flows again. A bottle filled with water that is kept in the deep freeze cracks for the same reason.
Specific heat capacity
Specific heat capacity is a measure of the amount of heat energy required to raise the temperature of a 1 kg mass of a substance by one degree Celsius (or one Kelvin). Its SI unit is J/kg K or J/kg °C.
The specific heat capacity of water being 4200 J/kg °C means that it takes 4200 Joules of energy to raise the temperature of 1 kilogram of water by 1 degree Celsius. Different objects have different specific heat capacity.
The formula for specific heat capacity (s) is:
Q = m s dt
Q=m s (T2−T1)
Q is the heat energy added or removed,
m is the mass of the substance,
c is the specific heat capacity
dt is the change in temperature
T1 is the initial temperature
T2 is the final temperature
Uses of specific heat capacity
- Water is used as a coolant in car radiators because water has a high specific heat capacity and it absorbs large amount of heat from the car’s engine, but the temperature of the water does not increase significantly.
- It is used as a hot compressor for soothing muscular pain because the high specific heat capacity of the hot water kept in the hot water bag releases its heat for a long time.
- The cycle of sea and land breezes due to the specific heat capacities of land and water, contributes to the relatively stable climate in coastal regions.
A sea breeze is a cool breeze that blows from the sea towards the land during the daytime. During the day, the land heats up faster than the sea because the land has a lower specific heat capacity than water. The air above the land becomes warmer and less dense, causing it to rise. Cooler, denser air from over the sea moves in to replace the rising warm air, creating a breeze that blows from the sea to the land.
A land breeze is a breeze that blows from the land towards the sea during the nighttime. At night, the land cools down faster than the sea because it loses heat more quickly due to its lower specific heat capacity. The air above the land becomes cooler and denser, causing it to sink and flow towards the sea, where the air is relatively warmer and less dense. This movement of air from the land to the sea creates a land breeze.
Calorimetry and its principle
The principle of calorimetry is based on the Law of Conservation of Energy, which states that energy cannot be created or destroyed, only transferred. In the context of calorimetry, this principle implies that the total heat lost by hot substances equals the total heat gained by cold substances, assuming no heat is lost to the surroundings.
If two substances are mixed in an insulated system, the heat lost by the hotter substance equals the heat gained by the cooler substance:
m1s1(T1−T f)=m2s2(T f−T2)
m1,m2 are the masses,
s1,s2 are the specific heat capacities,
T1,T2 are the initial temperatures,
T f is the final equilibrium temperature.
Thermometer and its types
A device used to measure the temperature of a body is called thermometer. When a body is heated, it expands and when it is cooled, it contracts. This is the working principle of a thermometer.
Types of thermometer
Laboratory thermometer: A laboratory thermometer is an instrument used to measure temperatures of different objects in laboratory. The scale in the laboratory thermometer ranges from -10°C to 110°C. Most laboratory thermometers are made of glass and contain either mercury or alcohol inside a thin capillary tube.
Clinical thermometer: The thermometer used to measure the temperature of the human body is called clinical thermometer. The typical range of a clinical thermometer is 35°C to 42°C (or 95°F to 107.6°F), which is suitable for measuring body temperature. Traditionally, clinical thermometers are mercury-in-glass devices, but many modern versions are digital, using electronic sensors to detect temperature.
Radiation thermometer: A radiation thermometer, commonly known as an infrared thermometer, measures temperature from a distance by detecting the infrared radiation emitted by an object or surface. The sensor produces electromagnetic signals and displays the temperature in the LCD panel.
Calibration of thermometer
Calibration of thermometer is the process of determining the scale in a thermometer.
Lower fixed point: The temperature of pure melting ice at the standard temperature and pressure is called the lower fixed point. It is 0°C or 273 K at standard atmospheric pressure.
Upper fixed point: The temperature of pure boiling water at the standard temperature and pressure is called the upper fixed point. It is 100°C or373 K at standard atmospheric pressure.
Temperature Scales
Celsius (°C)
- Freezing Point of Water: 0°C
- Boiling Point of Water: 100°C
Fahrenheit (°F)
- Freezing point of Water: 32°F
- Boiling Point of Water: 212°F
Kelvin (K)
- Freezing Point of Water: 273.15 K
- Boiling Point of Water: 373.15 K
Relation among Celsius, Fahrenheit and Kelvin scales
